6. Phosphoric acid, H3PO4, is one of the main ingredients of soft drinks, detergents, and fertilizers. It can be prepared with a series of reactions:
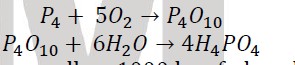
Let us say we allow 1000 kg of phosphorus to react with oxygen in the tank to yield 90% of tetra phosphorus decoxide (P4O10). In the second step of the reaction, we react it with water to yield 97% of H3PO4. How much in kilogram of H3PO4. How much in kilogram of H3PO4 that was produced after series of reactions
I AM STUDENT
Sir appreciate your work but can you please also add mdcat szambu past paper!
“Thank you, Aeman Rehan, for visiting MDCAT1.com! We sincerely appreciate your time and interest in our website. Yes, we will upload Szambu Papers shortly.”
sir really really appreciate your hard work… thankyou sooo much for this
I am facing a problem … NUMS 2014-2018 past papers are not opening… could you plz tell me what to do?
also I have a request … please add SZAMBU past papers also …